Style Sampler
Layout Style
Clinical Trials
Clinical Trials
Cancer Care Associates of York is dedicated in providing innovative oncology care for our community through participation in clinical trials. We are involved in a multitude of clinical trials, including screening, diagnostic and treatment trials across most cancer types and benign hematological processes. Our staff are well trained in Food and Drug Administration (FDA) regulations and are certified in Good Clinical Practice. Furthermore, we have potential access to cutting-edge clinical trial programs based on precision medicine with our partnership with the Tempus Time Trial Program.
Below, we invite you to learn more about our practice and our clinical studies.
Learn More (by clicking on the image below):
Understanding Clinical Trials:
1: Interventional Clinical Trials:
A study in which participants are assigned to a group that receives one or more intervention/treatment (or no interventions) that researchers can evaluate the effects and safety of interventions on biomedical or health-related outcomes. This generally involves medication, psychotherapy, a new device or a new approach to surgery or radiation. Assignments are assigned by study protocol. Participants may receive diagnostic, therapeutic, or other types of interventions.
2: Observational Trials:
A study in which participants are observed or certain outcomes are measured. There is no attempt made to affect the outcome or course of treatment.
3: Registry Trials:
A study that uses an organized system to collected information from an electronic medical record to evaluate a specific outcome for a population defined by a particular disease, condition, or exposure, that serves as a predetermined scientific, clinical or policy purpose.
Phases of Clinical Trials:
Clinical Trials have four interventional phases. You may see the names written as Phase I, II, III or IV or Phase 1, 2, 3, or 4. Below is what each means:
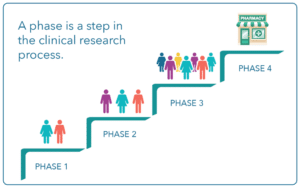
Phase 1 or (Phase I)
Experiment drug or treatment in a small group of people for the first time. Researchers are looking at the treatments safety to determine a safe dose range and to identify side effects.
Population: 20-100
Phase 2 or (Phase II)
Experimental drug or treatment given in a large group of people. Researchers use this phase to confirm its effectiveness and to further evaluate the safety of the drug or treatment.
Population: Up to several hundreds
Phase 3 or (Phase III)
Experimental study drug or treatment given in a larger population setting. Researchers use this to confirm its effectiveness, monitor side effects, compare to already approved treatments and to collect information that will allow the experimental drug or treatment to be used safely.
Population: 300-3,000
Phase 4 or (Phase IV)
This phase is used post-marketing after treatment is approved for use by the FDA. This helps aid in providing additional information including treatment or drugs risks, benefits, and best use.
Population: Several thousands
What Does it Mean to Participate?
Participation in any trial means that you have volunteered to participate in the trial after all information has been explained to you by your Oncologist and research team. This means that you may withdraw your consent from the trial at any time. Some reasons why you could choose to participate in the research studies:
Representation: Importance of Diversity in Clinical Research Trials:
Clinical trials are research studies that involve human volunteers to evaluate medical products. It is important that we ensure that the study population has diverse representation. The FDA have focused on this in advancing health equity. Although clinical trials should represent a variety of backgrounds, that is not always the case. Our mission at Cancer Care Associates of York is to make sure that our entire community have the opportunity to be involved in advanced research.